Key Takeaways
- Patients with desmoid tumors seek both disease control and symptom improvement
- For progressive, morbid, or symptomatic desmoid tumors, systemic therapies are recommended as a first-line treatment option according to the NCCN Guidelines® and DTWG Guideline1,2,*,†,‡
- There is one FDA-approved treatment option for desmoid tumors
- According to the NCCN Guidelines: In general, surgery is not considered a first-line treatment option for desmoid tumors, except in certain situations if agreed upon by a multidisciplinary tumor board2
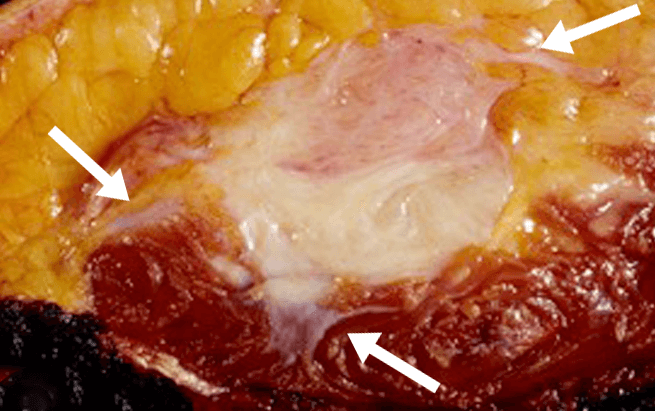
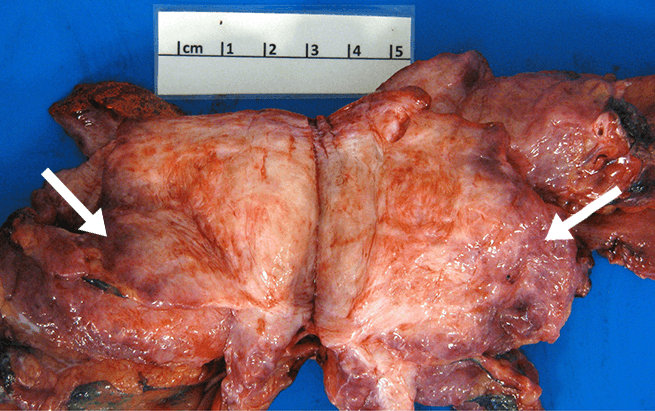
- Although they do not metastasize, desmoid tumors are associated with local recurrence rates ranging from 24% to 77% after surgical resection, regardless of margin status3,4,§
- Desmoid tumor recurrence risk remains high even with clean margins: various independent studies report desmoid tumor recurrence rates after complete surgical resection of 15% to 30%.5-8 Some studies suggest that the majority of tumors recur within 5 years5,6,9
- The NCCN Guidelines and DTWG Guideline recommend engaging a multidisciplinary care team with experience in desmoid tumors1,2
NCCN Guidelines also recommend ablation/embolization and definitive radiation therapy as first-line treatment options for progressive, morbid, or symptomatic desmoid tumors for certain patients.2
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of 33 leading cancer centers devoted to patient care, research, and education. The development of the NCCN Guidelines is an ongoing and iterative process based on a critical review of the best available evidence and derivation of recommendations by a multidisciplinary panel of experts in the field of cancer.2
The Desmoid Tumor Working Group (DTWG) consists of more than 90 sarcoma experts, patients, and patient advocates from around the world. The DTWG includes representatives from all disciplines involved in the management of desmoid tumors, including pathology, molecular biology, radiology, orthopedic surgery, surgical oncology, radiotherapy, medical oncology, and supportive care.1
Based on retrospective, observational data. Factors associated with local recurrence postsurgery include tumor location, age of the participant, tumor size, margin status, and prior recurrence.5,10
Management Options
NCCN Guidelines recommend that patients having tumors that are progressing, symptomatic, or impairing or threatening in function be offered therapy with the decision based on the location of the tumor and the potential morbidity of the therapeutic option.2 In addition to reduction of tumor growth, treatment considerations should include reducing symptoms and improving functioning and quality of life.12-14
To help with the diagnosis and management of desmoid tumors, explore the additional resources, clinical papers, and downloadable discussion tips in the Resources & Expert Videos section.
CT, computed tomography; FDA, US Food and Drug Administration; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network® (NCCN®).
- Kasper B, Baldini EH, Bonvalot S, et al; Desmoid Tumor Working Group. Current management of desmoid tumors: a review. JAMA Oncol. Accessed July 26, 2024. https://jamanetwork.com/journals/jamaoncology/article-abstract/2820212.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed August 9, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg. 1989;210(6):765-769.
- Skubitz KM. Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc. 2017;92(6):947-964.
- Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347-353.
- Peng PD, Hyder O, Mavros MN, et al. Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann Surg Oncol. 2012;19(13):4036-4042.
- Janssen ML, van Broekhoven DL, Cates JM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg. 2017;104(4):347-357.
- Nuyttens JJ, Rust PF, Thomas CR Jr, Turrisi AT 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88(7):1517-1523.
- Ballo MT, Zagars GK, Pollack A, et al. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158-167.
- Tsagozis P, Stevenson JD, Grimer R, Carter S. Outcome of surgery for primary and recurrent desmoid-type fibromatosis. A retrospective case series of 174 patients. Ann Med Surg (Lond). 2017;17:14-19.
- Joglekar SB, Rose PS, Sim F, Okuno S, Petersen I. Current perspectives on desmoid tumors: the Mayo Clinic approach. Cancers (Basel). 2011;3(3):3143-3155.
- Husson O, Younger E, Dunlop A, et al. Desmoid fibromatosis through the patients’ eyes: time to change the focus and organisation of care? Support Care Cancer. 2019;27(3):965-980.
- Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682-693.
- Timbergen MJM, van de Poll-Franse LV, Grünhagen DJ, et al. Identification and assessment of health-related quality of life issues in patients with sporadic desmoid-type fibromatosis: a literature review and focus group study. Qual Life Res. 2018;27(12):3097-3111.
- Kasper B, Baldini EH, Bonvalot S, et al; Desmoid Tumor Working Group. Current management of desmoid tumors: a review [supplementary online content]. JAMA Oncol. Accessed July 26, 2024. https://jamanetwork.com/journals/jamaoncology/article-abstract/2820212.
- Bonvalot S, Desai A, Coppola S, et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol. 2012;23(suppl 10):x158-x166.
- Lewis JJ, Boland PJ, Leung DHY, et al. The enigma of desmoid tumors. Ann Surg. 1999;229(6):866-872.
- Dafford K, Kim D, Nelson A, Kline D. Extraabdominal desmoid tumors. Neurosurg Focus. 2007;22(6):E21.
- Foo W, Lazar AJ. Pathology of desmoid tumors. In: Litchman C, ed. Desmoid Tumors. Springer; 2012. Accessed July 26, 2024. https://doi.org/10.1007/978-94-007-1685-8_3.
- Obeidin F, Alexiev B. Fibromatosis-desmoid. PathologyOutlines.com website. Accessed July 26, 2024. https://www.pathologyoutlines.com/topic/softtissuefibromatosisdeep.html.
- Penel N, Cesne AL, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125-131.
- Newman ET, Lans J, Kim J, et al. PROMIS function scores are lower in patients who underwent more aggressive local treatment for desmoid tumors. Clin Orthop Relat Res. 2020;478(3):563-577.
- Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785-1791.
- Fernandez MM, Bell T, Tumminello B, et al. Disease and economic burden of surgery in desmoid tumors: a review. Expert Rev Pharmacoecon Outcomes Res. 2023;23(6):607-618.
- Kasper B, Baumgarten C, Garcia J, et al. Desmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399-2408.
- Penel N, Chibon F, Salas S. Adult desmoid tumors: biology, management and ongoing trials. Curr Opin Oncol. 2017;28(10):2399-2408.