Patients with desmoid tumors seek both disease control and symptom improvement
- Desmoid tumors are locally aggressive, potentially morbid tumors of the soft tissues, with a tendency to infiltrate surrounding structures1-3
- Sometimes referred to as aggressive fibromatosis or desmoid fibromatosis, these mesenchymal tumors can be serious, debilitating and, in rare cases when vital organs are impacted, they can be life-threatening2,4,5
- Although they do not metastasize, desmoid tumors are associated with local recurrence rates ranging from 24% to 77% after surgical resection, regardless of margin status6,7,*
- Desmoid tumor recurrence risk remains high even with clean margins: various independent studies report desmoid tumor recurrence rates after complete surgical resection of 15% to 30%.8-11 Some studies suggest that the majority of tumors recur within 5 years8,9,12
Learn about surgery considerations
Based on retrospective, observational data. Factors associated with local recurrence postsurgery include tumor location, age of the participant, tumor size, margin status, and prior recurrence.8,13
Pathogenesis of desmoid tumors
- The pathogenesis of desmoid tumors is thought to be linked to dysregulated wound healing after trauma, such as childbirth, injury, or invasive surgery7,14-16
- Growth factors released during wound healing that promote beta-catenin activation may lead to recurrence of desmoid tumors14,17
Hear from a medical oncologist
Understanding Desmoid Tumors
Watch Dr. Riedel, medical oncologist from Duke Health and Duke Cancer Institute, provide an overview of desmoid tumors, including pathophysiology, clinical presentations, and impact on patients.
ICD-10-CM codes for desmoid tumors
Location-specific ICD-10-CM codes for desmoid tumors went into effect on October 1, 2023.18 The codes help enable healthcare providers to appropriately document diagnoses of patients with desmoid tumors and may help improve patient care.
Location-specific ICD-10-CM diagnosis codes for desmoid tumors can be incorporated into your clinical practice.

Knowing how to identify desmoid tumor progression can lead to timely initiation of appropriate treatment
Progression can be symptomatic and/or radiographic. Symptomatic progression may precede radiographic progression.19 In fact, evidence of pain can be a prognostic indicator of progression and can be associated with worse outcomes.19-21
In a study conducted at Memorial Sloan Kettering Cancer Center, 58% of patients with desmoid tumors converted from active surveillance to first-line treatment without radiographic tumor progression.22,†
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommendations for initiating treatment:23,‡,§
- Symptoms
- Impairing or threatening in function
- Tumor growth documented on imaging (e.g., MRI or CT)
See desmoid tumor radiology and pathology
Desmoid Tumor Working Group (DTWG) Guideline recommendations for initiating treatment:24,||
- Threatening to function or quality of life
- Life-threatening
- Persistent tumor growth¶
Learn about treatment options after early identification of desmoid tumor progression.
Primary or recurrent desmoid tumor patients (n=160) were identified retrospectively from an institutional database. Among the patients on initial observation for whom serial MRIs were available, there were 14 of 24 (58%) who underwent active treatment that did not experience tumor growth as defined by RECIST criteria.22
A course of ongoing observation is an appropriate option even for patients with disease progression, if the patient is minimally symptomatic and the anatomical location of the tumor is not critical. For tumors that are symptomatic, or impairing or threatening in function, patients should be offered therapy with the decision based on the location of the tumor and potential morbidity of the therapeutic option.23
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of 33 leading cancer centers devoted to patient care, research, and education. The development of the NCCN Guidelines® is an ongoing and iterative process based on a critical review of the best available evidence and derivation of recommendations by a multidisciplinary panel of experts in the field of cancer.23
The Desmoid Tumor Working Group (DTWG) consists of more than 90 sarcoma experts, patients, and patient advocates from around the world. The DTWG includes representatives from all disciplines involved in the management of desmoid tumors, including pathology, molecular biology, radiology, orthopedic surgery, surgical oncology, radiotherapy, medical oncology, and supportive care.24
Tumor size increase at >2 consecutive follow-up appointments; threatening quality of life, life, or function; or posing a potential risk to the feasibility or effectiveness of first-line treatment.24
Burden of Disease
Calling desmoid tumors “benign” is misleading
- Because of their histology and inability to metastasize, desmoid tumors are sometimes described as “benign.”5,25 However, that description can understate their potential impact on patients’ daily lives
- “Benign” can also suggest a manageable course of treatment, which may not be true for your patients5
- The true borders of desmoid tumors may not be identifiable as they can infiltrate surrounding tissue, compressing muscles, nerves, and vessels, and complicating attempts to obtain clean margins without leading to morbidity7,14,26
See images of tumor specimens post-resection - Patients may experience severe pain, limited function and mobility, and compromised quality of life25,27
- As desmoid tumors locally invade the bodies of patients, the physical toll can feel almost impossible to escape, and is often magnified by psychological, emotional, social, and even professional burdens25,28,29

“Let’s describe this disease as it actually is. Because it isn’t benign. It doesn’t metastasize. But it is life altering. It’s life changing.”
DeAnn, a real person living with a desmoid tumor
See how desmoid tumors may infiltrate the lives of patients in the video below
Patients with desmoid tumors report a variety of physical and psychological burdens27
In many patients, pain severity and burden of disease can lead to anxiety and depression.25,27,30
Data from a Memorial Sloan Kettering/Desmoid Tumor Research Foundation patient-reported outcome (PRO) validation study included patients with desmoid tumors (n=31, age range 20-68, 77% female). Patients participated in 60-minute qualitative phone interviews to provide their perspectives on disease symptoms and impact on their quality of life. The majority of the patients in this study were symptomatic (84%). Tumor site and type varied across patients. The concepts discussed during interviews were used to develop a draft patient-reported outcome scale, which was further refined in cognitive interviews of additional patients with desmoid tumors (n=15).27
Incidence and Risk Factors

There are approximately 1000 to 1650 annual cases in the United States31-33

Most patients are diagnosed between 20-44 years of age32

Female-to-male ratio is ~2-3:17,32,34

Recent pregnancy, injury, or surgery may increase risk15,16
Patients with familial adenomatous polyposis (FAP) have ~850-fold higher risk of developing desmoid tumors than the general population35 Read more about FAP
FAP is associated with desmoid tumors
Familial adenomatous polyposis (FAP) is an inherited syndrome resulting from germline mutations in the APC gene. FAP is linked to colorectal polyps that can lead to colorectal cancer.26 Desmoid tumors have been reported to occur in 10% to 12% of patients with FAP.2,35,36 Patients with FAP are at much higher risk of developing desmoid tumors than the general population.26
FAP-associated desmoid tumors are predominantly intra-abdominal (80%), usually within the mesentery. In addition, prior colectomy is associated with subsequent development of desmoid tumors, which represent a significant cause of morbidity in patients with FAP.2,26
TUMOR SITE
EST. FREQUENCY26
COMMON SYMPTOMS AND COMPLICATIONS
Intra-abdominal(including mesentery)
20%
Compression may cause pain, cachexia, malaise, abdominal distention, or obstruction of the intestines or ureters26,38,39
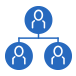
Intra-abdominal desmoid tumor40
This contrast-enhanced CT scan shows an unresectable, sporadic, mesenteric desmoid tumor (white arrow) in a female patient aged 30 years. The desmoid tumor is surrounding the superior mesenteric vessels (white arrowhead). The tumor’s multiple tendrils are threatening the bowel. After 3 months of observation, the patient presented with a bowel obstruction.
Abdominal Wall
16%
Large tumors may cause tissue stretching, blood vessel compression, and bowel or bladder displacement41
Lower Extremities
16%
Limited mobility, pain, muscle stiffness, or deformity 26,42
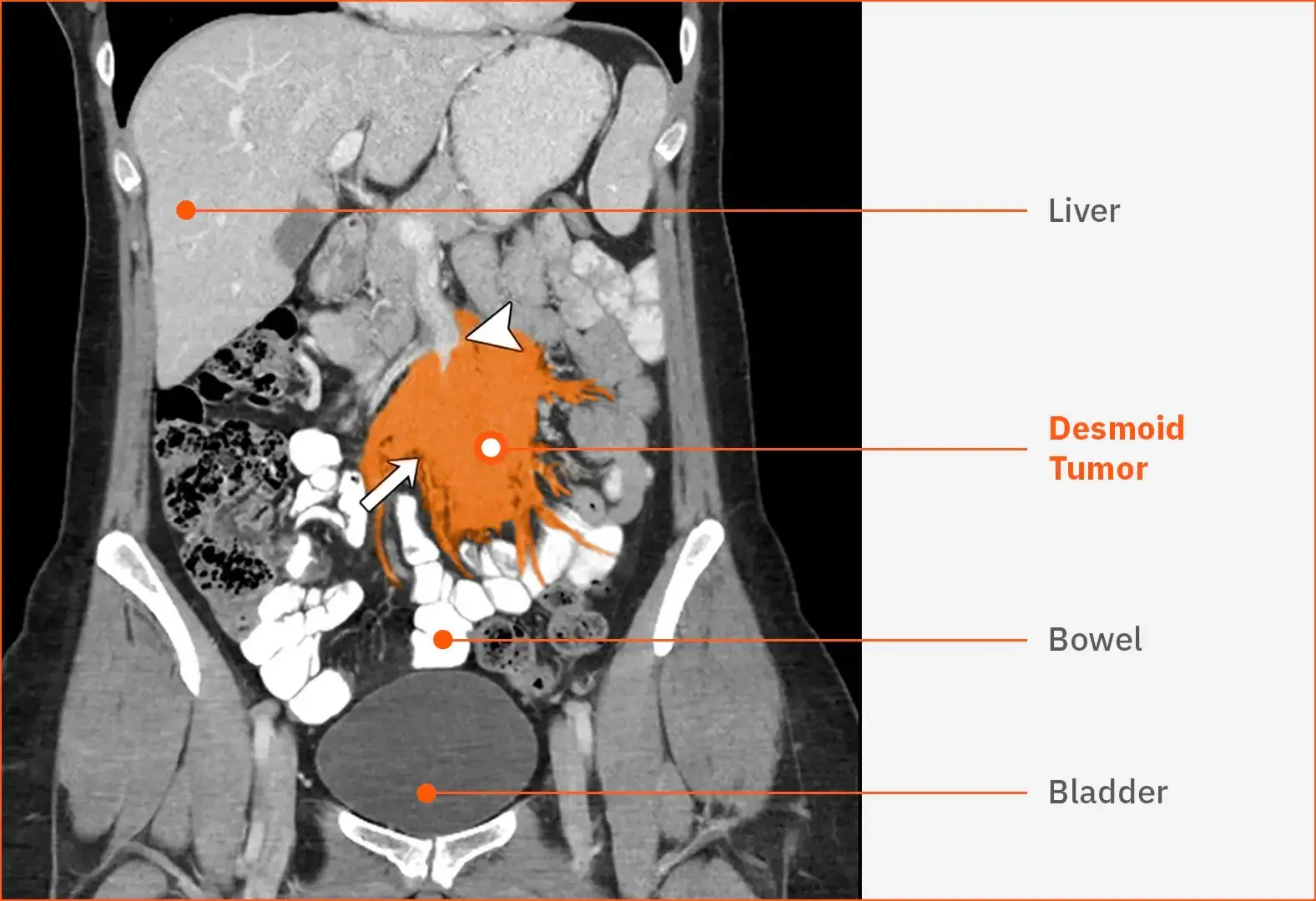
Multifocal desmoid tumors in the leg40
This T1-weighted MRI shows multiple, unresectable, sporadic desmoid tumors (white arrows) in the right leg of a female patient aged 40 years. The heterogeneous masses are infiltrating the calf region posteromedially.
Chest Wall
15%
Dyspnea, dysphagia, pleural invasion, rib or spinal involvement, bone erosion, and pain26,38,43,44
Upper Extremities
14%
Restricted mobility, muscle and ligament involvement, limb weakness, deformity, or pain26,45
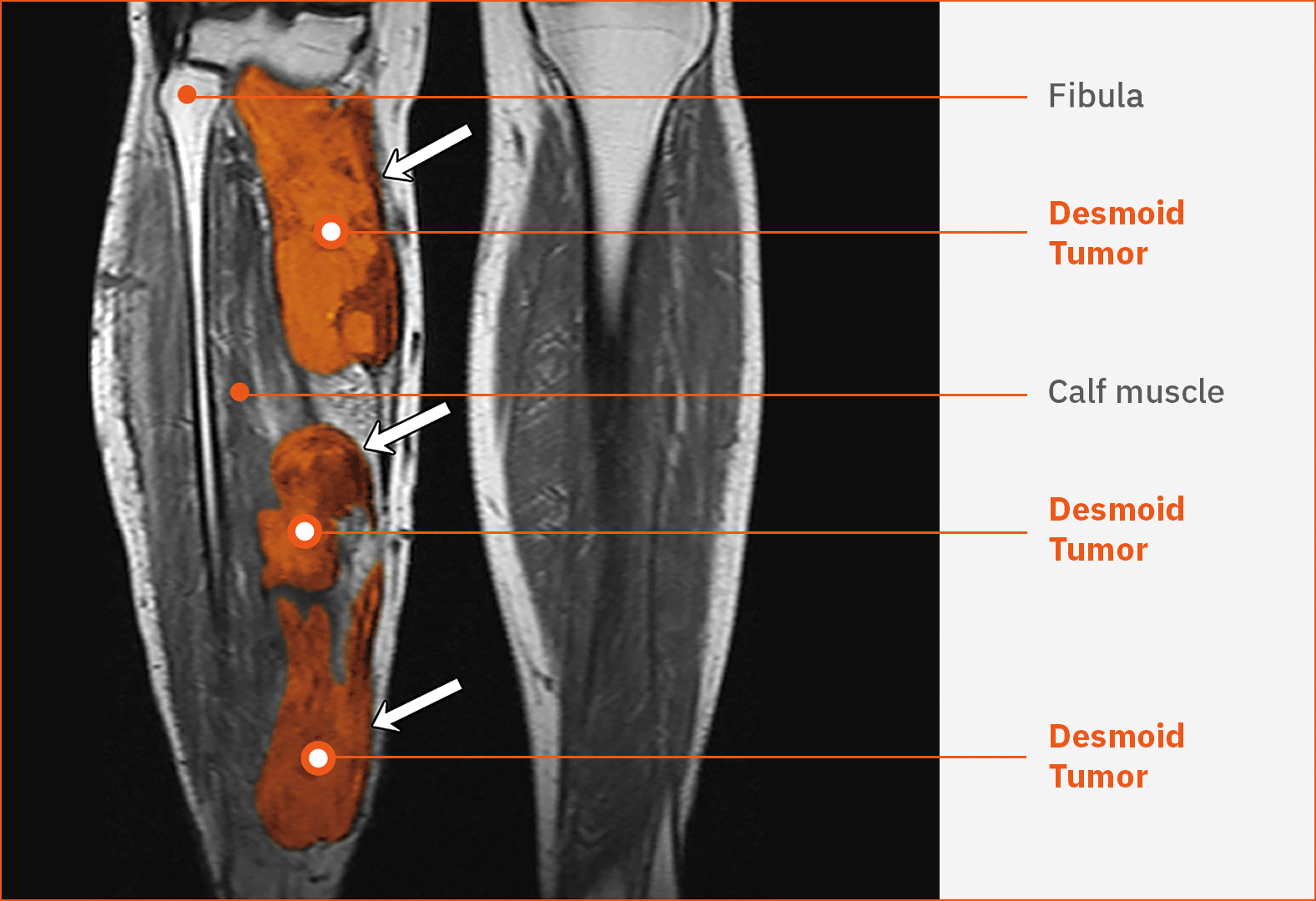
Desmoid tumor in the upper arm40
This contrast-enhanced T1-weighted MRI shows an unresectable, sporadic desmoid tumor (white arrow) in the right triceps region in a male patient aged 22 years.
Head and Neck
8%
Pain, neurologic deficit, proximity to vital structures, including mortality risk from vascular or airway restriction46
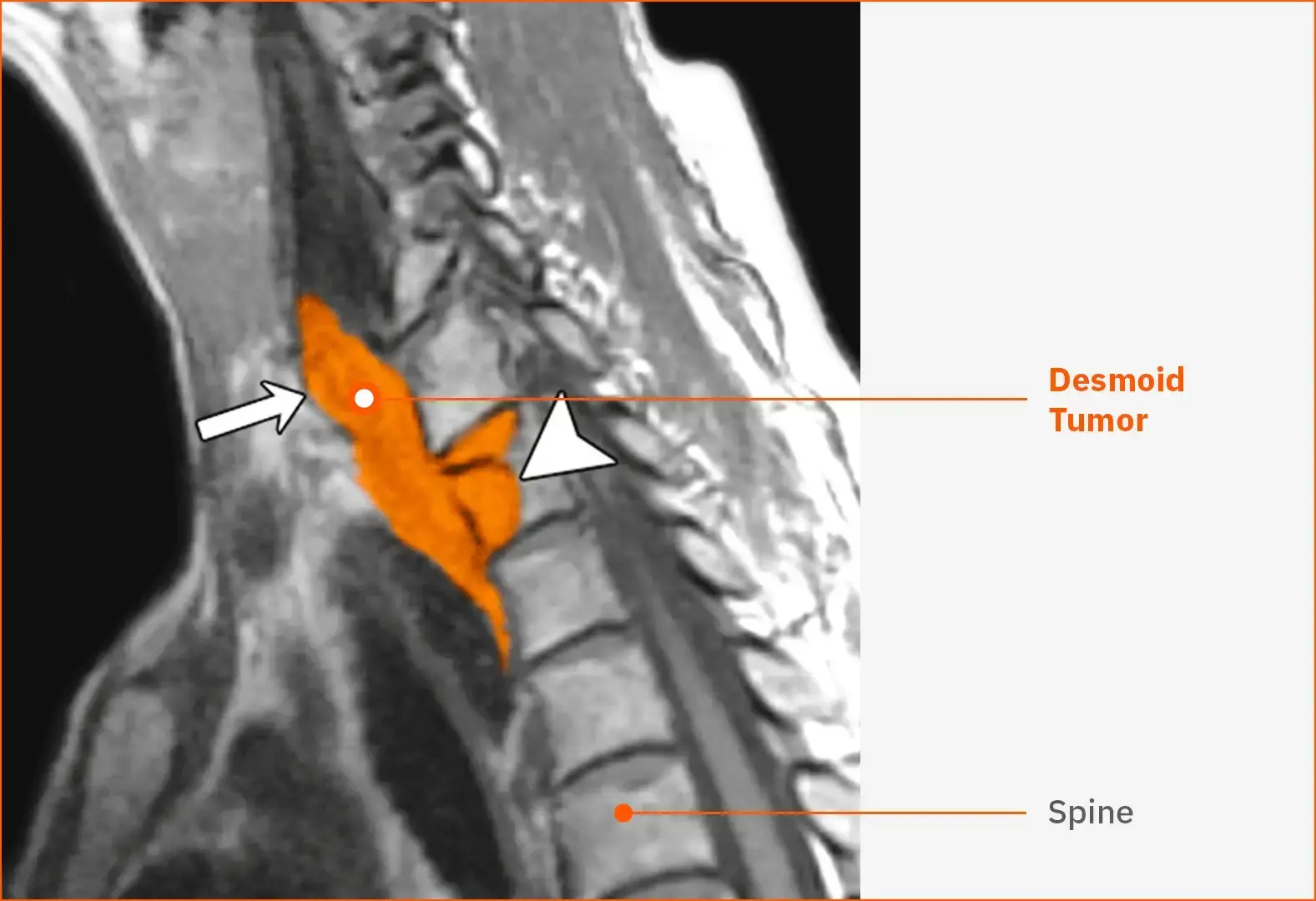
Desmoid tumor in the neck40
This T1-weighted MRI shows a recurrent desmoid tumor (white arrow) in the lower neck and throat of a female patient aged 52 years. The tumor is extending into the vertebral body (arrowhead).
Other
11%
Symptoms and complications dependent on the location5,26,27
CT, computed tomography; FDA, US Food and Drug Administration; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network® (NCCN®); RECIST, Response Evaluation Criteria in Solid Tumors.
- Kasper B, Baumgarten C, Garcia J, et al. Desmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma Patients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399-2408.
- Penel N, Chibon F, Salas S. Adult desmoid tumors: biology, management and ongoing trials. Curr Opin Oncol. 2017;29(4):268-274.
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021;113(2):70-84.
- Kasper B, Baldini EH, Bonvalot S, et al; Desmoid Tumor Working Group. Current management of desmoid tumors: a review [supplementary online content]. JAMA Oncol. Accessed July 26, 2024. https://jamanetwork.com/journals/jamaoncology/article-abstract/2820212.
- Joglekar SB, Rose PS, Sim F, Okuno S, Petersen I. Current perspectives on desmoid tumors: the Mayo Clinic approach. Cancers (Basel). 2011;3(3):3143-3155.
- Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg. 1989;210(6):765-769.
- Skubitz KM. Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc. 2017;92(6):947-964.
- Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347-353.
- Peng PD, Hyder O, Mavros MN, et al. Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann Surg Oncol. 2012;19(13):4036-4042.
- Janssen ML, van Broekhoven DL, Cates JM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg. 2017;104(4):347-357.
- Nuyttens JJ, Rust PF, Thomas CR Jr, Turrisi AT 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88(7):1517-1523.
- Ballo MT, Zagars GK, Pollack A, et al. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158-167.
- Tsagozis P, Stevenson JD, Grimer R, Carter S. Outcome of surgery for primary and recurrent desmoid-type fibromatosis. A retrospective case series of 174 patients. Ann Med Surg (Lond). 2017;17:14-19.
- Bonvalot S, Desai A, Coppola S, et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol. 2012;23(suppl 10):x158-x166.
- Fiore M, Coppola S, Cannell AJ, et al. Desmoid-type fibromatosis and pregnancy: a multi-institutional analysis of recurrence and obstetric risk. Ann Surg. 2014;259(5):973-978.
- Lopez R, Kemalyan N, Moseley HS, Dennis D, Vetto RM. Problems in diagnosis and management of desmoid tumors. Am J Surg. 1990;159(5):450-453.
- Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293(2):267-274.
- Centers for Medicare & Medicaid Services 2024 ICD-10-CM codes. Centers for Medicare & Medicaid Services Web Site. Accessed July 26, 2024. https://www.cms.gov/medicare/coding-billing/icd-10-codes/2024-icd-10-cm.
- Cuomo P, Scoccianti G, Schiavo A, et al. Extra-abdominal desmoid tumor fibromatosis: a multicenter EMSOS study. BMC Cancer. 2021;21(1):437.
- Penel N, Bonvalot S, Le Deley MC, et al. Pain in desmoid-type fibromatosis: prevalence, determinants and prognosis value. Int J Cancer. 2023;153(2):407-416.
- Quintini C, Ward G, Shatnawei A, et al. Mortality of intra-abdominal desmoid tumors in patients with familial adenomatous polyposis: a single center review of 154 patients. Ann Surg. 2012;255(3):511-516.
- Cassidy MR, Lefkowitz RA, Long N, et al. Association of MRI T2 signal intensity with desmoid tumor progression during active observation: a retrospective cohort study. Ann Surg. 2020;271(4):748-755.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed August 9, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Kasper B, Baldini EH, Bonvalot S, et al; Desmoid Tumor Working Group. Current management of desmoid tumors: a review. JAMA Oncol. Accessed July 26, 2024. https://jamanetwork.com/journals/jamaoncology/article-abstract/2820212.
- Husson O, Younger E, Dunlop A, et al. Desmoid fibromatosis through the patients’ eyes: time to change the focus and organisation of care? Support Care Cancer. 2019;27(3):965-980.
- Constantinidou A, Scurr M, Judson I, Litchman C. Clinical presentation of desmoid tumors. In: Litchman C, ed. Desmoid Tumors. Springer; 2012:chap 2. Accessed July 26, 2024. https://www.researchgate.net/publication/226455135.
- Gounder MM, Maddux L, Paty J, Atkinson TM. Prospective development of a patient-reported outcomes instrument for desmoid tumors or aggressive fibromatosis. Cancer. 2020;126(3):531-539.
- Timbergen MJM, van de Poll-Franse LV, Grünhagen DJ, et al. Identification and assessment of health-related quality of life issues in patients with sporadic desmoid-type fibromatosis: a literature review and focus group study. Qual Life Res. 2018;27(12):3097-3111.
- Mercier KA, Hernandez L, Boulanger V, et al; Desmoid Tumor Research Foundation; National Organization for Rare Disorders (NORD); Trio Health Analytics. Quality of life and tumor location in patients with desmoid tumors: data from the desmoid tumor research foundation natural history study. ASCO® Meeting Library. Accessed July 26, 2024. https://meetinglibrary.asco.org/record/173143/abstract.
- Paty J, Maddux L, Gounder MM. Prospective development of a patient reported outcomes (PRO) tool in desmoid tumors: a novel clinical trial endpoint. ASCO® Meeting Library. Accessed July 26, 2024. https://ascopubs.org/doi/10.1200/JCO.2017.35.15_suppl.11022.
- Orphanet Report Series. Prevalence and incidence of rare diseases: bibliographic data. Accessed July 26, 2024. https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf.
- van Broekhoven DLM, Grünhagen DJ, den Bakker MA, van Dalen T, Verhoef C. Time trends in the incidence and treatment of extra-abdominal and abdominal aggressive fibromatosis: a population-based study. Ann Surg Oncol. 2015;22(9):2817-2823.
- U.S. Department of Commerce. News Blog. U.S. population estimated at 332,403,650 on Jan. 1, 2022. Accessed July 26, 2024. https://www.commerce.gov/news/blog/2022/01/us-population-estimated-332403650-jan-1-2022#:~:
- Penel N, Coindre JM, Bonvalot S, et al. Management of desmoid tumours: a nationwide survey of labelled reference centre networks in France. Eur J Cancer. 2016;58:90-96.
- Gurbuz AK, Giardiello FM, Petersen GM, et al. Desmoid tumours in familial adenomatous polyposis. Gut. 1994;35(3):377-381.
- Bertario L, Russo A, Sala P, et al. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int J Cancer. 2001;95(2):102-107.
- Penel N, Cesne AL, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125-131.
- Shinagare AB, Ramaiya NH, Jagannathan JP, et al. A to Z of desmoid tumors. AJR Am J Roentgenol. 2011;197(6):W1008-W1014.
- Tchangai BK, Tchaou M, Alassani F, et al. Giant abdominopelvic desmoid tumour herniated trough perineum: a case report. J Surg Case Rep. 2021;2021(8):rjab295.
- Braschi-Amirfarzan M, Keraliya AR, Krajewski KM, et al. Role of imaging in management of desmoid-type fibromatosis: a primer for radiologists. Radiographics. 2016;36(3):767-782.
- Koshariya M, Shukla S, Khan Z, et al. Giant desmoid tumor of the anterior abdominal wall in a young female: a case report. Case Rep Surg. 2013;2013:780862.
- McDonald ES, Yi ES, Wenger DE. Best cases from the AFIP: extraabdominal desmoid-type fibromatosis. Radiographics. 2008;28(3):901-906.
- Abrão FC, Waisberg DR, Fernandez A, et al. Desmoid tumors of the chest wall: surgical challenges and possible risk factors. Clinics (Sao Paulo). 2011;66(4):705-708.
- Xie Y, Xie K, Gou Q, He J, Zhong L, Wang Y. Recurrent desmoid tumor of the mediastinum: a case report. Oncol Lett. 2014;8(5):2276-2278.
- Scaramussa FS, Castro UB. Desmoid tumor in hand: a case report. SM J Orthop. 2016;2(3):1036.
- Baranov E, Hornick JL. Soft tissue special issue: fibroblastic and myofibroblastic neoplasms of the head and neck. Head Neck Pathol. 2020;14(1):43-58.
- Ingley KM, Klein R, Theobalds N, et al. High prevalence of persistent emotional distress in desmoid tumor. Psycho-Oncology. 2020;29(2):311-320.


